What is Biliary Tract Cancer?
Biliary tract cancer is a term that includes cholangiocarcinoma and gall bladder cancer. Cholangiocarcinoma is a type of cancer that originates in the bile ducts. The bile ducts are thin tubes that transport bile from the liver to the small intestine, where it aids in the digestion of fats. Bile is stored in the gall bladder. Biliary tract cancer can occur in any part of the biliary system, including the gall bladder, intrahepatic (inside the liver), perihilar (where the bile ducts exit the liver), and distal (outside the liver) regions.
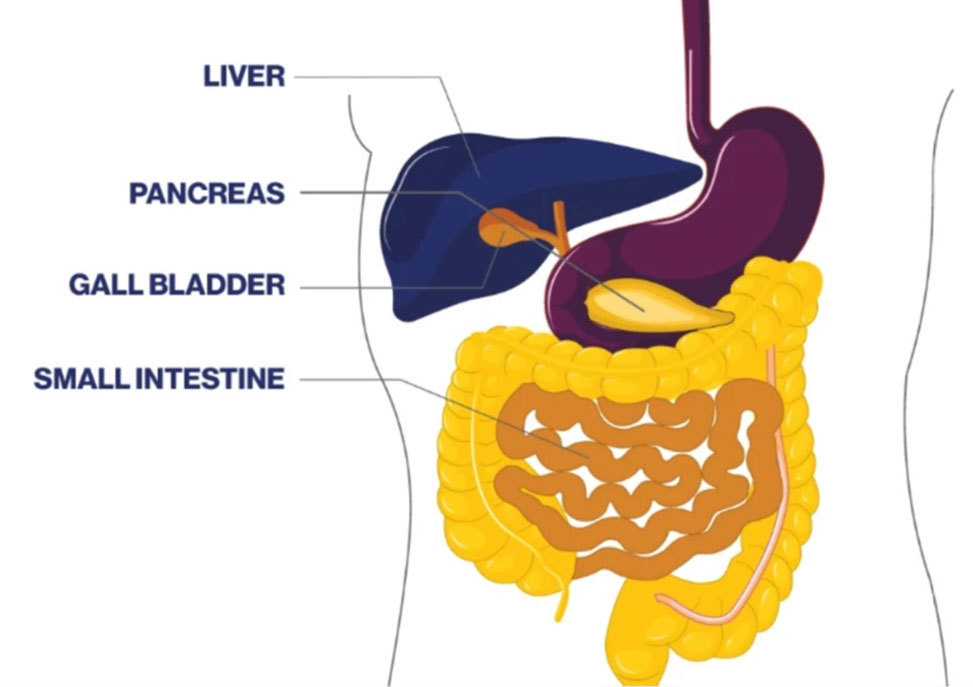
Biliary tract cancer is relatively rare compared to other types of cancer; it is often not diagnosed until a very advanced stage and can be aggressive and difficult to treat. Common symptoms of biliary tract cancer may include jaundice (yellowing of the skin and eyes), abdominal pain, unexplained weight loss, itching, and changes in stool or urine color.
Are Bile Duct Cancer and Biliary Tract Cancer the same things?
Bile duct cancer is a type of biliary tract cancer also known as cholangiocarcinoma. Cholangiocarcinoma originates in the bile ducts, which are the thin tubes that carry bile from the liver to the small intestine. Biliary cancer is a broader term that encompasses cholangiocarcinoma and gall bladder cancer.
How is Biliary Tract Cancer related to Gallbladder Cancer?
Gallbladder cancer is another malignancy within the biliary system. The gallbladder is a small organ located beneath the liver that stores bile produced by the liver.
Gallbladder cancer specifically refers to malignancies that develop in the tissues of the gallbladder. While it is a distinct type of cancer, gallbladder cancer is a kind of biliary tract cancer.
What is the drug being investigated?
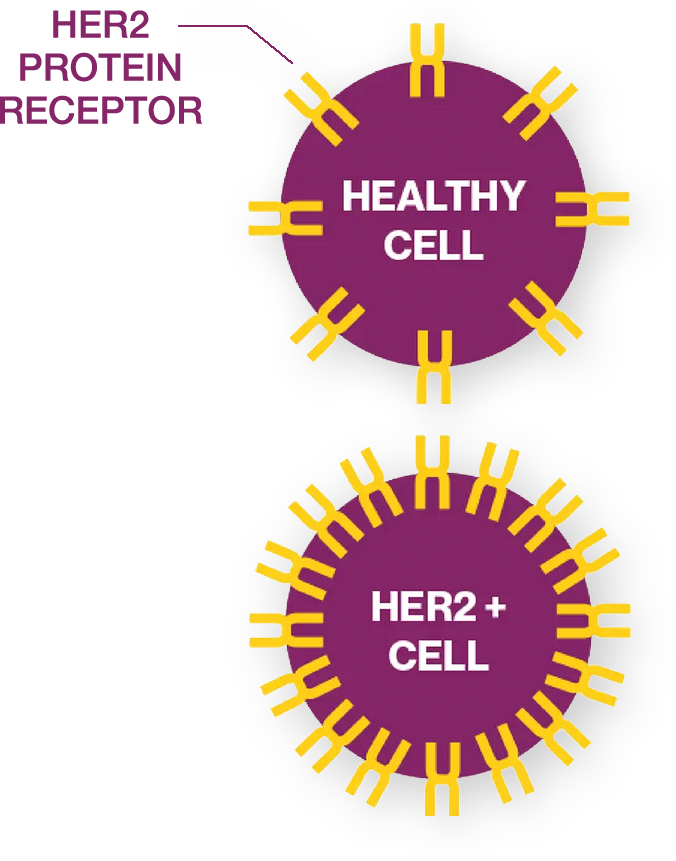
The open-label, randomized Phase 3 clinical trial is investigating zanidatamab. Zanidatamab is an investigational medication that works by blocking the HER2 signal in the body while removing the HER2 protein from the cell surface. HER2 signaling tells cancer cells to survive, grow, and multiply. So, blocking this signal and removing HER2 protein from cancer cells may treat tumors. In addition, we are studying Zanidatamab’s potential to activate the immune system to attack tumors, which may also help to treat the cancer.
In this trial, zanidatamab is not being studied alone, but in combination with standard treatments for biliary tract cancer.


What do I need to know about the study?
This biliary tract cancer study will involve approximately 286 participants from around the world. Participants will receive either zanidatamab plus standard of care treatments or standard of care treatment alone. Standard of care treatment include some medications which will only be given for a defined period of time (about 6 months) and others, including zanidatamab, that may be given for as long as it is continuing to help the participant.
Participants in the study will receive the study medicine, as well as study-related visits, tests and assessments, at no cost. Participants can stop taking part in the clinical trial at any time without giving a reason. Caregivers of participants may also be reimbursed for some study-related expenses, such as costs associated with travel and hotel.
The research team will be able to explain more about what the study will involve, and it is up to you to decide if you want to take part. Participation in this study is voluntary. Your decision to participate or not participate will have no effect on the medical care you receive now or in the future.
See If You Are Eligible
If you meet these requirements, you may be eligible to participate.
Learn more to be put in touch with trial staff.
Must have a confirmed diagnosis of HER2 positive Biliary Tract Cancer

May only have received up to 2 prior treatment cycles of standard therapy
Must be male or female over 18 years of age
Are you Eligible to Participate in the study?
Here is a list of key Inclusion/Exclusion Criteria to participate in the study:
Key Inclusion Criteria
- Participants must have a confirmed diagnosis of HER2 positive Biliary Tract Cancer, including Gallbladder Cancer, Intrahepatic Cholangiocarcinoma, or Extrahepatic Cholangiocarcinoma that cannot be effectively treated with surgery or other local (in the body) treatments such as radiation therapy or direct tumor destruction.
- Participants may only have received up to 2 prior treatment cycles of standard therapy.
- Participants must be male or female over 18 years of age (or the legal age of adulthood per country-specific regulations).
- Females of childbearing potential must have a negative pregnancy test result.
- Females of childbearing potential and males with a partner of childbearing potential must be willing to use 2 methods of birth control.
Key Exclusion Criteria
- Participants may not have received prior treatment with a HER2-targeted agent or checkpoint inhibitors. The only exception is if they have received two or fewer doses of durvalumab or pembrolizumab as part of the treatment that is allowed prior to study entry (described in the inclusion criteria).
- Participants must not use high dose systemic corticosteroids or other medications that suppress the immune system. There are some exceptions to this, please consult with your doctor about what is allowed.
- Participants must not have brain metastases.
- Participants may not have severe chronic or active infections, a history of allogeneic organ transplantation, active prior autoimmune inflammatory conditions, or interstitial lung disease or non-infectious pneumonitis.
- Participants may not have participated in another clinical trial with an investigational medicinal product within the last 3 months.
- Females who are breastfeeding are not eligible to participate.
- Participants may not have any other medical, social, or psychosocial factors that, in the opinion of the investigator, could impact safety or compliance with study procedures.
Frequently Asked Questions
What is a study?
A study (also called a clinical trial) is a medical investigation that helps to answer important questions about an investigational medication, including if the medication works for a certain condition and how safe and tolerable the medication is. All medications must be tested in clinical research studies before they can be approved by regulatory authorities and prescribed to patients.
What is a study?
Why are studies important?
What is the purpose of the study?
Will compensation for time and travel be provided?
Is there a cost to participate?
- You will receive study-related care from a team of experienced doctors and nurses throughout the study.
- All study-related visits, tests, assessments, and investigational medication will be provided at no cost to you.
What else do I need to consider?
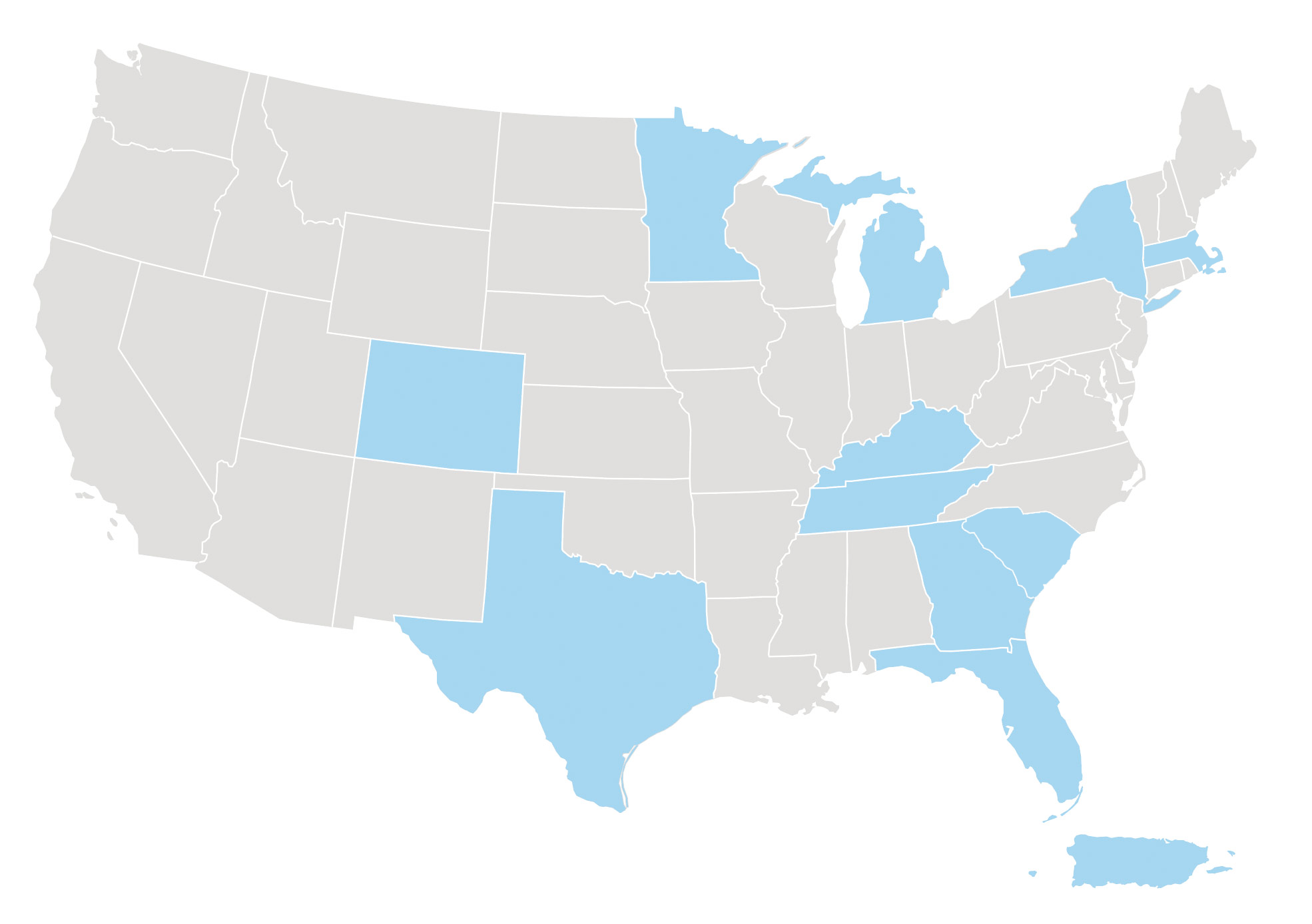
Where are the study sites located?
The Study Is Taking Place Now!
Investigator Locator
Contact a participating site to learn if this trial may be appropriate for you or your loved ones. Please reach out to us at ClinicalTrialDisclosure@jazzpharma.com to learn more about our sites near you.
For site location, please contact ClinicalTrialDisclosure@jazzpharma.com
or visit clinicaltrials.gov (and search “NCT06282575” or click here).
Norton Cancer Institute
SITE CONTACT:
Michael Driscoll, MD
Principal Investigator
Email:
GI-NCIResearch@nortonhealthcare.org
Phone:
+1 502-629-2500
University of Michigan
SITE CONTACT:
Vaibhav Sahai
Principal Investigator
UM Cancer Answerline Nurses
Study Coordinator
Email:
CancerAnswerLine@med.umich.edu
Phone:+1 800-865-1125
Henry Ford Health System
Contact information: clinicaltrialdisclosure@jazzpharma.com
James J. Harding Memorial Sloan Kettering Cancer
SITE CONTACT:
James J. Harding, MD
Principal Investigator
Raymond Teer
Study Coordinator
Email: teerr@mskcc.org
Amin Yaqubie
Research Manager
Email: yaqubiea@mskcc.org
Elizabeth Warner
Research Manager
Email: warnere@mskcc.org
Phone:
+1 332-456-7016
Laura and Isaac Perlmutter Cancer Center at NYU Langone - Ambulatory Care Center
SITE CONTACT:
Spencer
Principal Investigator
clinicaltrialdisclosure@jazzpharma.com
Tufts Medical Center
SITE CONTACT:
Nugent
Principal Investigator
clinicaltrialdisclosure@jazzpharma.com
Saint Francis Cancer Center
SITE CONTACT:
Yang
Principal Investigator
clinicaltrialdisclosure@jazzpharma.com
Sarah Cannon Research Institute (SCRI) Oncology
SITE CONTACT:
Meredith Pelster, MD
Principal Investigator
Phone:
+1 844-482-4812
James J. Harding Memorial Sloan Kettering Cancer
Puerto Rico Medical Center
Second Floor, Barrio Monacillos
Rio Piedras, Puerto Rico, USA 00935
SITE CONTACTS:
Karina Arocho-Gonzalez, MD
Principal Investigator
Email:karina.arocho@panoncologytrials.com
Isamar Alicea
Study Coordinator
Email:isamar.alicea@panoncologytrials.com
Phone: +1 787-407-3333
Website: panoncologytrials.com
AdventHealth Hematology and Oncology
Location: Orlando, Florida, US 32804
Contact information: clinicaltrialdisclosure@jazzpharma.com
Rocky Mountain Cancer Centers, LLP
Location: Lone Tree, Colorado, US 80124
Contact information: clinicaltrialdisclosure@jazzpharma.com
Texas Oncology - DFW
Contact information: clinicaltrialdisclosure@jazzpharma.com
The Univerrsity of Texas MD Anderson Cancer Center
Contact information: clinicaltrialdisclosure@jazzpharma.com
Minnesota Oncology Hematology, P.A.
Contact information: clinicaltrialdisclosure@jazzpharma.com
