Trial Purpose
The purpose of this study is to compare the efficacy of zanidatamab and CisGem (cisplatin plus gemcitabine) with or without the addition of a programmed death protein 1/ligand 1 (PD-1/L-1) inhibitor versus CisGem with or without a PD-1/L1 inhibitor in adult participants.
What is Biliary Tract Cancer?
Biliary tract cancer is a term that includes cholangiocarcinoma and gall bladder cancer. Cholangiocarcinoma is a type of cancer that originates in the bile ducts. The bile ducts are thin tubes that transport bile from the liver to the small intestine, where it aids in the digestion of fats. Bile is stored in the gall bladder. Biliary tract cancer can occur in any part of the biliary system, including the gall bladder, intrahepatic (inside the liver), perihilar (where the bile ducts exit the liver), and distal (outside the liver) regions.
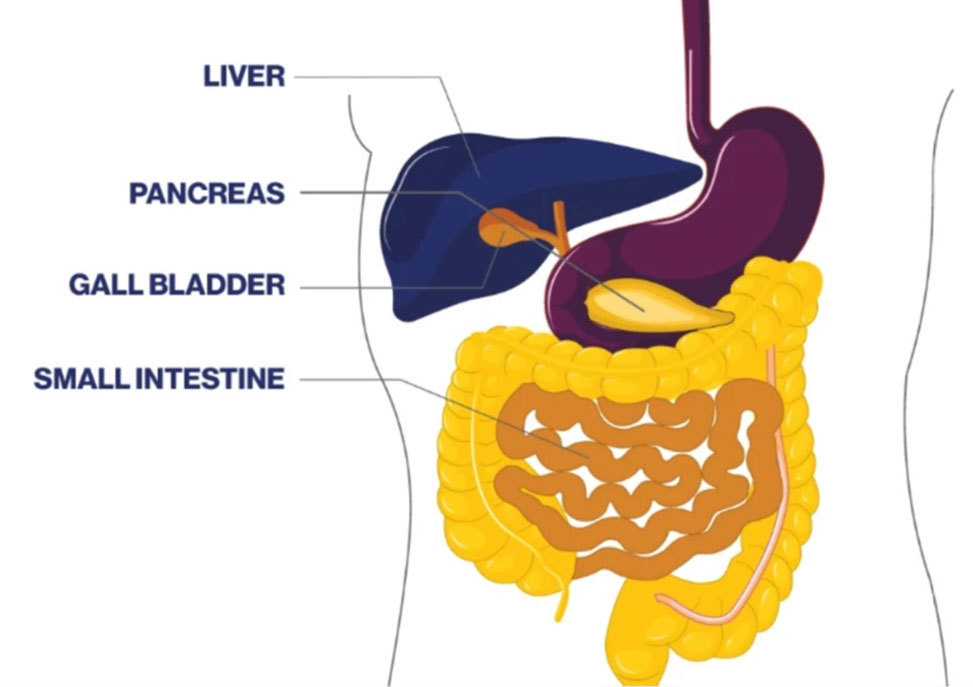
Biliary tract cancer is relatively rare compared to other types of cancer; it is often not diagnosed until a very advanced stage and can be aggressive and difficult to treat. Common symptoms of biliary tract cancer may include jaundice (yellowing of the skin and eyes), abdominal pain, unexplained weight loss, itching, and changes in stool or urine color.
Are Bile Duct Cancer and Biliary Tract Cancer the same things?
Bile duct cancer is a type of biliary tract cancer also known as cholangiocarcinoma. Cholangiocarcinoma originates in the bile ducts, which are the thin tubes that carry bile from the liver to the small intestine. Biliary cancer is a broader term that encompasses cholangiocarcinoma and gall bladder cancer.
How is Biliary Tract Cancer related to Gallbladder Cancer?
Gallbladder cancer is another malignancy within the biliary system. The gallbladder is a small organ located beneath the liver that stores bile produced by the liver.
Gallbladder cancer specifically refers to malignancies that develop in the tissues of the gallbladder. While it is a distinct type of cancer, gallbladder cancer is a kind of biliary tract cancer.
What is the drug being investigated?
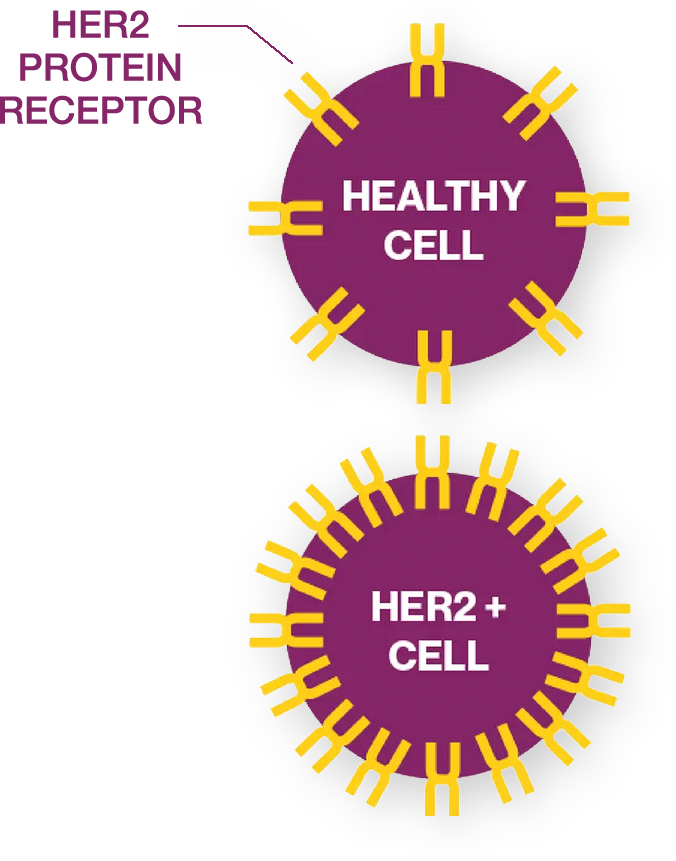
The open-label, randomized phase 3 clinical trial is investigating zanidatamab. Zanidatamab is an investigational medication that works by blocking the HER2 signal in the body while removing the HER2 protein from the cell surface. HER2 signaling tells cancer cells to survive, grow, and multiply. So, blocking this signal and removing HER2 protein from cancer cells may treat tumors. In addition, we are studying zanidatamab’s potential to activate the immune system to attack tumors, which may also help to treat the cancer.
In this trial, zanidatamab is not being studied alone, but in combination with standard treatments for biliary tract cancer.


Information about the study
This biliary tract cancer study will involve approximately 286 participants from around the world. Participants will receive either zanidatamab plus standard of care treatments or standard of care treatment alone. Standard of care treatment include some medications which will only be given for a defined period of time (about 6 months) and others, including zanidatamab, that may be given for as long as it is continuing to help the participant.
Participants in the study will receive the study medicine, as well as study-related visits, tests and assessments, at no cost. Participants can stop taking part in the clinical trial at any time without giving a reason. Caregivers of participants may also be reimbursed for some study-related expenses, such as costs associated with travel and hotel.
Participation in the study is voluntary and participation decisions have no impact on medical care you receive now or in the future.
Frequently Asked Questions
What is a study?
A study (also called a clinical trial) is a medical investigation that helps to answer important questions about an investigational medication, including if the medication works for a certain condition and how safe and tolerable the medication is. All medications must be tested in clinical research studies before they can be approved by regulatory authorities and prescribed to patients.
What is a study?
Why are studies important?
What is the purpose of the study?
Where are the study sites located?
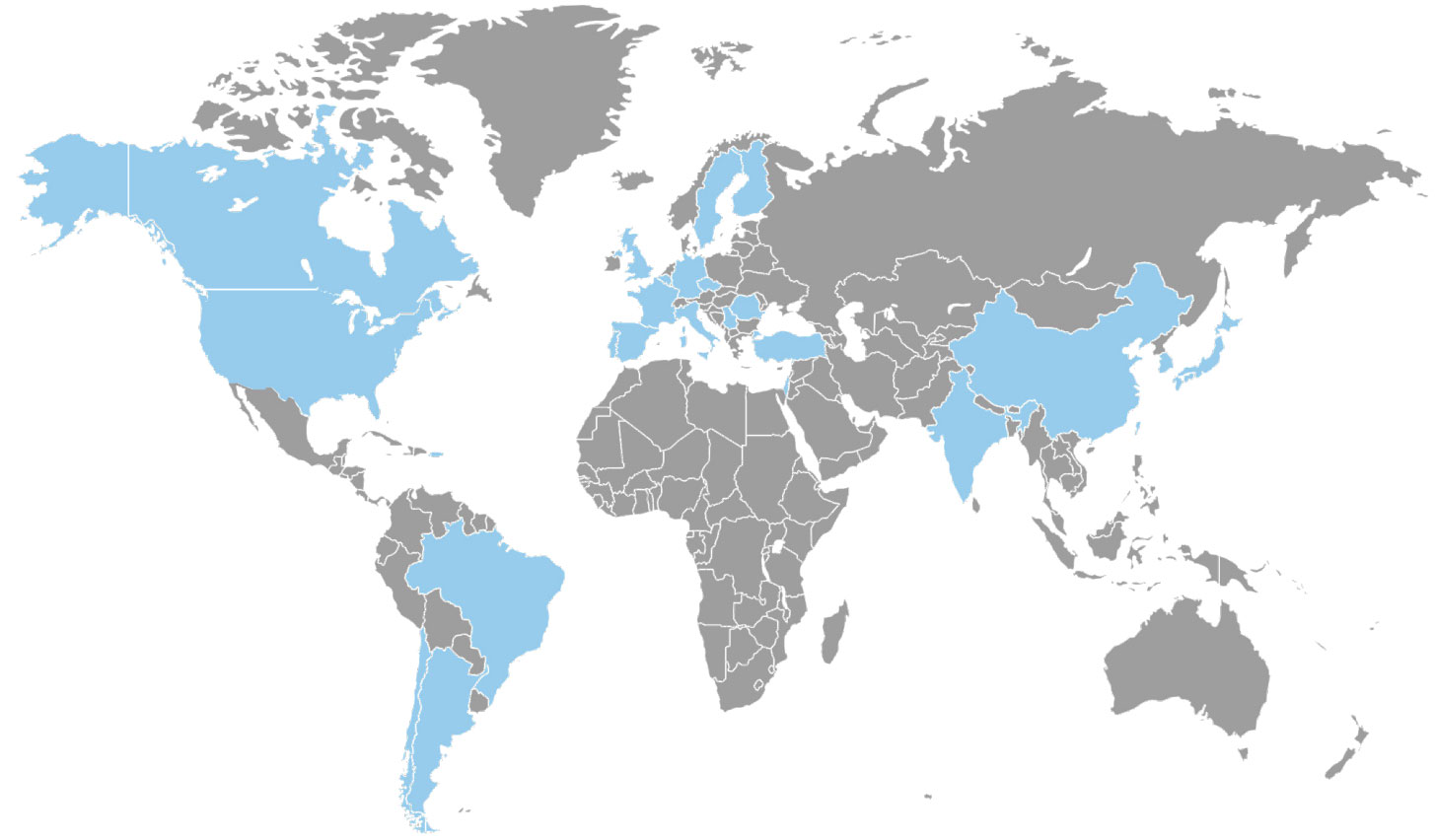
The Study is Taking Place Now!
The study is taking place in the following countries: Argentina, Belgium, Brazil, Canada, Chile, China, Czech Republic, Finland, France, Germany, India, Israel, Italy, Japan, Portugal, Romania, Serbia, S. Korea, Spain, Sweden, Taiwan, Turkey, UK, and USA.
For site location, please contact ClinicalTrialDisclosure@jazzpharma.com or visit clinicaltrials.gov (and search “NCT06282575” or click here).
Additional site and trial information is available for Patients and Healthcare Providers in the United States.
Argentina
Buenos Altes Locations:
Hospital Italiano de Buenos Aires
Location: Ciudad Autónoma de Buenos Aires (CABA) Argentina, C1199ABB
Contact information: clinicaltrialdisclosure@jazzpharma.com
Córdoba Locations:
Centro Médico Privado CEMAIC
Location: Córdoba, Argentina, X5008HHW
Contact information: clinicaltrialdisclosure@jazzpharma.com
Jujuy Locations:
Fundación ARS Médica
Location: San Salvador De Jujuy, Jujuy, Argentina, 4600
Contact information: clinicaltrialdisclosure@jazzpharma.com
Río Negro Locations:
Centro de Investigaciónes Clínicas – Clínica Viedma
Location: Viedma, Río Negro, Argentina, R8500ACE
Contact information: clinicaltrialdisclosure@jazzpharma.com
Santa Fe Locations:
Hospital Provincial Del Centenario
Location: Rosario, Santa Fe, Argentina, 2000
Contact information: clinicaltrialdisclosure@jazzpharma.com
Instituto Médico de la Fundación Estudios Clínicos
Location: Rosario, Santa Fe, Argentina, S2013DTC
Contact information: clinicaltrialdisclosure@jazzpharma.com
Tucumán Locations:
CAIPO Centro para la Atención Integral del Paciente Oncológico
Location: San Miguel De Tucumán, Tucumán, Argentina, 4000
Contact information: clinicaltrialdisclosure@jazzpharma.com
Centro Médico en Oncología Clínica EXELSUS S.R.L
Location: San Miguel De Tucumán, Tucumán, Argentina, 4000
Contact information: clinicaltrialdisclosure@jazzpharma.com
Belgium
Antwerpen Locations:
Imelda VZW
Location: Bonheiden, Belgium, 2820
Contact information: clinicaltrialdisclosure@jazzpharma.com
UZ Antwerpen
Location: Edegem, Belgium, 2650
Contact information: clinicaltrialdisclosure@jazzpharma.com
Vlaams-Brabant Locations:
UZ Leuven
Location: Leuven, Belgium, 3000
Contact information: clinicaltrialdisclosure@jazzpharma.com
Brazil
Hospital Nossa Senhora Da Conceição/Centro Integrado de Pesquisa em Oncologia CIPO-GHC
Location: Porto Alegre, Brazil, 91350-200
Contact information: clinicaltrialdisclosure@jazzpharma.com
Rio de Janeiro Locations:
Instituto Nacional de Cancer – INCA
Location: Rio de Janeiro, Brazil, 20230-130
Contact information: clinicaltrialdisclosure@jazzpharma.com
Rio Grande do Norte Locations:
Liga Norte Riograndense Contra O Cancer
Location: Natal, Brazil, 59062-000
Contact information: clinicaltrialdisclosure@jazzpharma.com
Rio Grande do Sul Locations:
Hospital de Clinicas de Porto Alegre – HCPA
Location: Porto Alegre, Brazil, 90035-903
Contact information: clinicaltrialdisclosure@jazzpharma.com
Irmandade Da Santa Casa de Misericordia de Porto Alegre – ISCMPA
Location: Porto Alegre, Brazil, 90020-170
Contact information: clinicaltrialdisclosure@jazzpharma.com
São Paulo Locations:
Fundação Faculdade Regional de Medicina de São José do Rio Preto (FUNFARME)
Location: São Paulo, Brazil, 15090-000
Contact information: clinicaltrialdisclosure@jazzpharma.com
Fundação Pio XII Hospital de Amor de Barretos
Location: Barretos, Brazil, 14.784-400
Contact information: clinicaltrialdisclosure@jazzpharma.com
Canada
London Health Sciences Centre
Location: London, Ontario Canada, NGA 5W9
Contact information: clinicaltrialdisclosure@jazzpharma.com
Princess Margaret Cancer Centre – University Health Network
Location: Toronto, Ontario Canada, M5G 2M9
Contact information: clinicaltrialdisclosure@jazzpharma.com
Chile
Araucanía Locations
James Lind Centro de Investigacion del Cancer
Temuco, Araucanía, Chile, 4800827
Libertador Gen Bernardo O Higgins Locations
Location: Rancagua, Libertador Gen Bernardo O Higgins, Chile, 2820000
Servicios Médicos URUMED SpA
Contact information: clinicaltrialdisclosure@jazzpharma.com
Los Lagos Locations
Clinica Puerto Montt
Puerto Montt, Los Lagos, Chile, 5507642
Contact information: clinicaltrialdisclosure@jazzpharma.com
Metropolitana Locations:
Biocinetic Ltda
Location: Santiago, Región-Metropolitana, Chile, 8320000
Contact information: clinicaltrialdisclosure@jazzpharma.com
Centro de Investigacion y Especialidades Medicas (CDIEM)
Location: Santiago, Región-Metropolitana, Chile, 7500859
Contact information: clinicaltrialdisclosure@jazzpharma.com
Fundación Arturo López Pérez (FALP)
Caglevic
Principal Investigator
Location: Santiago, Chile, 7500921
Contact information: clinicaltrialdisclosure@jazzpharma.com
Hospital Clinico Universidad de Chile
Villanueva Olivares
Principal Investigator
Location: Santiago, Región-Metropolitana, Chile, 8380494
Contact information: clinicaltrialdisclosure@jazzpharma.com
Oncovida
Romero Sanchez
Principal Investigator
Location: Santiago, Chile, 7500994
Contact information: clinicaltrialdisclosure@jazzpharma.com
Czech Republic
Fakultni nemocnice Hradec Kralove, Klinika onkologie a radioterapie
Location: Hradec Kralove, Czech Republic, 50005
Contact information: clinicaltrialdisclosure@jazzpharma.com
Fakultni nemocnice v Motole, Onkologicka klinika
Location: Praha 5, Czech Republic, 150 00
Contact information: clinicaltrialdisclosure@jazzpharma.com
Fakultní nemocnice Brno, Interní hematologická a onkologická klinika
Location: Brno, Czech Republic, 625 00
Contact information: clinicaltrialdisclosure@jazzpharma.com
Finland
Comprehensive Cancer Center, Helsinki University Hospital
Helsinki, Finland 290
Contact information: clinicaltrialdisclosure@jazzpharma.com
Kuopio University Hospital
Kuopio, Finland 70029
Contact information: clinicaltrialdisclosure@jazzpharma.com
Docrates Cancer Center
Helsinki, Finland 180
Contact information: clinicaltrialdisclosure@jazzpharma.com
France
CHU Brest Hôpital de la Cavale Blanche
Brest, France 29200
Contact information: clinicaltrialdisclosure@jazzpharma.com
CHU de Bordeaux – Hôpital Haut Leveque
Pessac, France 33604
Contact information: clinicaltrialdisclosure@jazzpharma.com
CHU de Toulouse – (Toulouse University Hospital Center)
Toulouse, France 31059
Contact information: clinicaltrialdisclosure@jazzpharma.com
CHU Poitiers
Poitiers, France 86000
Contact information: clinicaltrialdisclosure@jazzpharma.com
CHU Estaing
Clermont-Ferrand, France 63100
Contact information: clinicaltrialdisclosure@jazzpharma.com
CHU Montepellier – Hôpital Saint Eloi
Montpellier, France 34000
Contact information: clinicaltrialdisclosure@jazzpharma.com
Gustave Roussy
Villejuif, France 94805
Contact information: clinicaltrialdisclosure@jazzpharma.com
Hospital Beaujon University
Clichy, France 92110
Contact information: clinicaltrialdisclosure@jazzpharma.com
Hopital Henri Mondor
Créteil, France 94010
Contact information: clinicaltrialdisclosure@jazzpharma.com
Institut de Cancérologie de l’Ouest Saint-Herblain
Cedex, France 44805
Contact information: clinicaltrialdisclosure@jazzpharma.com
CHU Besancon
Besançon, France 25030
Contact information: clinicaltrialdisclosure@jazzpharma.com
Germany
Charité – Universitätsmedizin Berlin – Campus Virchow Klinikum, Medizinische Klinik mit Schwerpunkt Hamatologie und Tumorimmunologie
Berlin, Germany 13353
clinicaltrialdisclosure@jazzpharma.com
Hessen Locations:
Krankenhaus Nordwest – Institut für Klinisch-Onkologische Forschung (IFK)
Frankfurt, Germany 60488
clinicaltrialdisclosure@jazzpharma.com
Baden-Wuttemberg Locations:
Universitätsklinikum Tübingen – Medizinische Universitätsklinik, Innere Medizin I
Tuebingen, Germany 72076
clinicaltrialdisclosure@jazzpharma.com
Nationales Centrum für Tumorerkrankungen (NCT) Heidelberg
Heidelberg, Germany 69120
clinicaltrialdisclosure@jazzpharma.com
Universitätsklinikum Freiburg – Klinik für Innere Medizin II,
Freiburg, Germany 79106
clinicaltrialdisclosure@jazzpharma.com
Bayern Locations:
LMU Universitätsklinikum München, Campus Großhadern, Medizinische
München, Germany 81377
clinicaltrialdisclosure@jazzpharma.com
Nieder-Sachsen Locations:
Medizinische Hochschule Hannover – Klinik Fur Gastroenterologie, Hepatologie, Infektiologie und Endokrinologie
Hannover, Germany 30625
clinicaltrialdisclosure@jazzpharma.com
Hamburg Locations:
Asklepios Tumorzentrum Hamburg, Asklepios Klinik Altona – Onkologie und Palliativmedizin mit Sektionen Hamatologie und Rheumatologie
Hamburg, Germany 22763
clinicaltrialdisclosure@jazzpharma.com
Sachsen Locations:
Technische Universität Dresden
Dresden, Germany 1307
clinicaltrialdisclosure@jazzpharma.com
Schleswig-Holstein Locations:
Universitätsklinikum Schleswig-Holstein – Campus Lübeck, Medizinische Klinik I (MKI)
Lubeck, Germany 23562
clinicaltrialdisclosure@jazzpharma.com
India
Rajiv Gandhi Cancer Institute And Research Centre
New Delhi, India 110085
clinicaltrialdisclosure@jazzpharma.com
Karnataka Locations
KLES Dr Prabhakar Kore Hospital and Medical Research Centre
Belagavi, Karnataka, India 590010
clinicaltrialdisclosure@jazzpharma.com
Maharashtra Locations
Chopda Medicare & Research Centre Pvt. Ltd – Magnum Heart Institute
Nashik, Maharashtra, India, 422005
clinicaltrialdisclosure@jazzpharma.com
Tata Memorial Hospital
Mumbai, Maharashtra, India, 4000012
clinicaltrialdisclosure@jazzpharma.com
New Delhi Locations
Max Super Speciality Hospital – Saket (a unit of Devki Devi foundation)
New Delhi, India, 110017
clinicaltrialdisclosure@jazzpharma.com
Telangana Locations
AIG Hospitals (A Unit of Asian Institute of Gastroenterology)
Hyderabad, Telangana, India 500032
clinicaltrialdisclosure@jazzpharma.com
Pi Health Cancer Hospital
Hyderabad, Telangana, India 500032
clinicaltrialdisclosure@jazzpharma.com
Uttar Pradesh Locations
Mahamana Pandit Madan Mohan Malviya Cancer Centre
Varanasi, Uttar Pradesh, India 221005
clinicaltrialdisclosure@jazzpharma.com
Israel
Rambam Health Care Campus
Principal Investigator: Maria Passhak
Coordinator: Liat Rapaport (Referral coordinator)
Email: O_trials@rambam.health.gov.il
Telephone: +97247776234
Jerusalem, Israel, 91120
The Hadassah University Medical Center, Ein Kerem Hospital
Principal Investigator: Ayala Hubert
Coordinator: Ilana Schoen
Email: ILAMACH@hadassah.org.il
Telephone: 053-8220805
Petah-Tikva, Israel, 49100
Rabin Medical Center, Bellinson Hospital
Principal Investigator: Salomon Stemmer
Coordinator: Michal Cohavi
Email: shmichalco1@clalit.org.il
Telephone: 054-5286262
Ramat Gan, HaMerkaz, Israel, 5265601
The Chaim Sheba Medical Center, Tel Hashomer
Principal Investigator: Dr. Ofer Margalit
Coordinator: Anna Ruth Rapoport
Email: Anna.Rapoport@sheba.health.gov.il
Telephone: +972-35305349
Tel Aviv, Israel, 6423906
Tel Aviv Sourasky Medical Center (Ichilov)
Principal Investigator: Professor Ravit Geva
Coordinator: Itay Eisenkot
Email: Oncologyresearch@tlvmc.gov.il
Telephone: 03-6977362
Italy
Istituto Clinico Humanitas
Rozzano, Italy 20089
clinicaltrialdisclosure@jazzpharma.com
Azienda Ospedaliera Universitaria Integrata Verona
Verona, Italy 37134
clinicaltrialdisclosure@jazzpharma.com
AOU dell’Universit~ degli Studi della Campania Luigi Vanvitelli
Napoli, Italy 80131
clinicaltrialdisclosure@jazzpharma.com
Azienda Ospedaliera Universitaria Integrata Verona
Verona, Italy 37134
clinicaltrialdisclosure@jazzpharma.com
Azienda Sanitaria Universitaria Friuli Centrale – P.O. S. Maria della Misericordia
Udine Italy, 33100
clinicaltrialdisclosure@jazzpharma.com
Azienda Ospedaliero – Universitaria Pisana
Pisa, Italy 56126
clinicaltrialdisclosure@jazzpharma.com
Fondazione IRCCS Istituto Nazionale per lo studio e la cura dei Tumori
Milano, Italy 20133
clinicaltrialdisclosure@jazzpharma.com
Instituo di Candiolo, Fondazione del Piemonte per l’Oncologia (IRCCS)
Candiolo, Torina, Italy 10060
clinicaltrialdisclosure@jazzpharma.com
IRCCS Ospedale Policlinico San Martino
Genova, Italy 16132
clinicaltrialdisclosure@jazzpharma.com
Veneto Institute of Oncology IOV – I.R.C.C.S.
Padova, Italy 35128
clinicaltrialdisclosure@jazzpharma.com
Japan
Aichi Locations:
Aichi Cancer Center
Nagoya-shi, Aichi, Japan 464-8681
clinicaltrialdisclosure@jazzpharma.com
Chiba Locations:
Chiba Cancer Center
Chiba-shi, Chiba, Japan 260-8717
clinicaltrialdisclosure@jazzpharma.com
National Cancer Center Hospital East
Kashiwa, Chiba, Japan 277-8577
clinicaltrialdisclosure@jazzpharma.com
Ehime Locations:
National Hospital Organization Shikoku Cancer Center
Matsuyama-Shi, Ehime, Japan 791-0280
clinicaltrialdisclosure@jazzpharma.com
Fukuoka Locations:
National Hospital Organization Kyushu Cancer Center
Fukuoka-shi, Fukuoka, Japan 811-1395
clinicaltrialdisclosure@jazzpharma.com
Hokkaidô Locations:
Hokkaido University Hospital
Sapporo-Shi, Hokkaidô, Japan 060-8648
clinicaltrialdisclosure@jazzpharma.com
Isikawa Locations:
Kanazawa University Hospital
Kanazawa-shi, Isikawa, Japan 9208641
clinicaltrialdisclosure@jazzpharma.com
Kagoshima Locations:
Kagoshima University Hospital
Kagoshima-shi, Kagoshima, Japan 890-8520
clinicaltrialdisclosure@jazzpharma.com
Kanagawa Locations:
Kanagawa Cancer Center
Yokohama-shi, Kanagawa, Japan 241-8515
clinicaltrialdisclosure@jazzpharma.com
Kyôto Locations:
Kyoto University Hospital
Kyoto City, Kyôto, Japan 606-8507
clinicaltrialdisclosure@jazzpharma.com
Mie Locations:
Mie University Hospital
Tsu-Shi, Mie, Japan 514-8507
clinicaltrialdisclosure@jazzpharma.com
Miyagi Locations:
Tohoku University Hospital
Sendai-Shi, Miyagi, Japan 980-8574
clinicaltrialdisclosure@jazzpharma.com
Niigata Locations:
Niigata University Medical and Dental Hospital
Niigata-shi, Niigata, Japan 951-8520
clinicaltrialdisclosure@jazzpharma.com
Ôsaka Locations:
Osaka University Hospital
Suita-Shi, Ôsaka, Japan 565-0871
clinicaltrialdisclosure@jazzpharma.com
Osaka Prefectural Hospital Organization Osaka International Cancer Institute
Osaka-shi, Ôsaka, Japan 541-8567
clinicaltrialdisclosure@jazzpharma.com
Tokyo Locations:
National Cancer Center Hospital
Chuo-Ku, Tokyo, Japan 104-0045
clinicaltrialdisclosure@jazzpharma.com
The Cancer Institute Hospital of Japanese Foundation For Cancer Research
Koto-Ku, Tokyo, Japan 135-8550
clinicaltrialdisclosure@jazzpharma.com
Kyorin University Hospital
Mitaka-shi, Tokyo, Japan 181-8611
clinicaltrialdisclosure@jazzpharma.com
The University of Tokyo Hospital
Bunkyo-ku, Tokyo, Japan 113-8655
clinicaltrialdisclosure@jazzpharma.com
Yamaguchi Locations:
Yamaguchi University Hospital
Ube-Shi, Yamaguchi, Japan 755-8505
clinicaltrialdisclosure@jazzpharma.com
Korea, Republic of
Seoul, Korea, Republic of, 2841
clinicaltrialdisclosure@jazzpharma.com
Samsung Medical Center
Seoul, Korea, Republic of, 6351
clinicaltrialdisclosure@jazzpharma.com
Seoul National University Hospital
Seoul, Korea, Republic of, 3080
clinicaltrialdisclosure@jazzpharma.com
Severance Hospital, Yonsei University Health System
Seoul, Korea, Republic of, 3722
clinicaltrialdisclosure@jazzpharma.com
Asan Medical Center
Seoul, Korea, Republic of, 5505
clinicaltrialdisclosure@jazzpharma.com
Korea University Guro Hospital
Seoul, Korea, Republic of, 8308
clinicaltrialdisclosure@jazzpharma.com
Pusan National University Hospital
Busan, South Korea 49241
clinicaltrialdisclosure@jazzpharma.com
Gyeonggi-do Locations
CHA Bundang Medical Center, CHA University
Bundang-Gu, Seongnam-Si, Gyeonggi-do, Korea, Republic of, 13496
clinicaltrialdisclosure@jazzpharma.com
National Cancer Center
Ilsandong-gu, Goyang-si, Gyeonggi-do, Korea, Republic of, 10408
clinicaltrialdisclosure@jazzpharma.com
Seoul National University Bundang Hospital
Seongnam-si, Gyeonggi-do, Korea, Republic of, 13620
clinicaltrialdisclosure@jazzpharma.com
Portugal
Lisboa, Portugal, 1400-038
clinicaltrialdisclosure@jazzpharma.com
Instituto Português de Oncologia Do Porto Francisco Gentil, EPE
Porto, Portugal, 4200-072
clinicaltrialdisclosure@jazzpharma.com
ULS de Coimbra, EPE – Hospitais da Universidade de Coimbra
Coimbra, Portugal, 3004-561
clinicaltrialdisclosure@jazzpharma.com
Unidade Local de Saude de Almada-Seixal, E.P.E. – Hospital Garcia de Orta
Almada, Portugal, 2805-267
clinicaltrialdisclosure@jazzpharma.com
Unidade Local de Saúde de São José – Hospital de Santo António dos Capuchos
Lisboa, Portugal, 1150-314
clinicaltrialdisclosure@jazzpharma.com
Puerto Rico
Pan American Center for Oncology Trials, LLC
Second Floor, Barrio Monacillos
Rio Piedras, Puerto Rico, USA 00935
Karina Arocho-Gonzalez, MD
Principal Investigator
Email:karina.arocho@panoncologytrials.com
Isamar Alicea
Study Coordinator
Email:isamar.alicea@panoncologytrials.com
Phone: +1 787-407-3333
Website: panoncologytrials.com
Romania
Institutul Clinic Fundeni
Bucharest, Romania 22328
clinicaltrialdisclosure@jazzpharma.com
Iasi Locations:
Centrul de Oncologie Euroclinic SRL
Iasi, Iasi, Romania 700106
clinicaltrialdisclosure@jazzpharma.com
Dolj Locations:
Centrul De Oncologie “SF. Nectarie” S.R.L.
Craiova, Dolj, Romania 200542
clinicaltrialdisclosure@jazzpharma.com
Bihor Locations:
Spital Clinic Judetean de Urgenta Bihor
Oradea, Bihor, Romania 410169
clinicaltrialdisclosure@jazzpharma.com
Serbia
Belgrade, Serbia 11040
clinicaltrialdisclosure@jazzpharma.com
Oncology Institute of Vojvodina, Internal Oncology Clinic
Sremska Kamenica, Serbia 21204
clinicaltrialdisclosure@jazzpharma.com
University Clinical Center of Serbia, Clinic for Gastroenterohepatology
Belgrade, Serbia 11000
clinicaltrialdisclosure@jazzpharma.com
Spain
Córdoba, Spain, 14004
clinicaltrialdisclosure@jazzpharma.com
Complejo Hospitalario Universitario de Santiago -Hospital Clinico Universitario de Santiago
Santiago de Compostela, A Coruna, Spain, 15706
clinicaltrialdisclosure@jazzpharma.com
Vall d’Hebron Institute of Oncology/Hospital Universitari de Vall d’Hebron
Barcelona, Spain, 8035
clinicaltrialdisclosure@jazzpharma.com
Hospital Universitario La Paz
Madrid, Spain, 28048
clinicaltrialdisclosure@jazzpharma.com
Hospital Universitario 12 de Octubre
Madrid, Spain, 28041
clinicaltrialdisclosure@jazzpharma.com
Hospital Universitario Fundacion Jimenez Diaz
Madrid, Spain, 28040
clinicaltrialdisclosure@jazzpharma.com
Hospital Regional Universitario de Malaga
Malaga, Spain, 29010
clinicaltrialdisclosure@jazzpharma.com
Clinica Universidad de Navarra
Pamplona, Navarra, Spain, 31008
clinicaltrialdisclosure@jazzpharma.com
Hospital Universitario Virgen Macarena
Sevilla, Spain, 41009
clinicaltrialdisclosure@jazzpharma.com
Hospital Universitario y Politecnico La Fe
Valencia, Spain, 46026
clinicaltrialdisclosure@jazzpharma.com
Hospital Universitario Miguel Servet
Zaragoza, Spain, 50009
clinicaltrialdisclosure@jazzpharma.com
Sweden
Malmö, Sweden 205 02
clinicaltrialdisclosure@jazzpharma.com
Onkologiska mottagningen, Skånes Universitetssjukhus Malmö
Huddinge, Sweden 14186
clinicaltrialdisclosure@jazzpharma.com
Onkologiska mottagningen, Sahlgrenska Universitetssjukhuset
Göteborg, Sweden 413 45
clinicaltrialdisclosure@jazzpharma.com
Taiwan
Kaohsiung, Taiwan 807
clinicaltrialdisclosure@jazzpharma.com
Chang Gung Memorial Hospital Kaohsiung Branch of the Chang Gung Medical Foundation
Kaohsiung, Taiwan 833
clinicaltrialdisclosure@jazzpharma.com
Chang Gung Memorial Hospital Linkou Branch of the Chang Gung Medical Foundation
Taoyuan, Taiwan 333
clinicaltrialdisclosure@jazzpharma.com
National Cheng Kung University Hospital
Tainan, Taiwan 704
clinicaltrialdisclosure@jazzpharma.com
National Taiwan University Cancer Center
Taipei, Taiwan 106
clinicaltrialdisclosure@jazzpharma.com
Turkey
Edirne, Turkey 22030
clinicaltrialdisclosure@jazzpharma.com
Hacettepe University Cancer Institute
Ankara, Sihhiye, Turkey 6230
clinicaltrialdisclosure@jazzpharma.com
Akdeniz University Medical Faculty Hospital
Antalya, Turkey 7070
clinicaltrialdisclosure@jazzpharma.com
Istanbul University Cerrahpasa Medical Faculty Hospital/Clinical Research Center
Istanbul, Fatih, Turkey 34096
clinicaltrialdisclosure@jazzpharma.com
United Kingdom
Manchester, England M20 4BX, United Kingdom
clinicaltrialdisclosure@jazzpharma.com
Mount Vernon Cancer Centre
London, England HA6 2RN, United Kingdom
clinicaltrialdisclosure@jazzpharma.com
Royal Marsden Hospital NHS Foundation Trust
Surrey, England SM2 5PT, United Kingdom
clinicaltrialdisclosure@jazzpharma.com
Royal Marsden Hospital NHS Foundation Trust
London, England SW3 6JJ, United Kingdom
clinicaltrialdisclosure@jazzpharma.com
Western General Hospital, Edinburgh Cancer Centre
Edinburgh, Scotland EH4 2XU, United Kingdom
clinicaltrialdisclosure@jazzpharma.com
United States
Colorado Locations:
Rocky Mountain Cancer Centers (Aurora) – USOR
Location: Denver, Colorado, US 80218
Contact information: clinicaltrialdisclosure@jazzpharma.com
Florida Locations:
AdventHealth Orlando
Location: Orlando, Florida, US 32804
Contact information: clinicaltrialdisclosure@jazzpharma.com
Georgia Locations:
Winship Cancer Institute
Location: Atlanta, Georgia, US 30322
Contact information: clinicaltrialdisclosure@jazzpharma.com
Kansas Locations:
The University of Kansas Cancer Center – Westwood
Location: Westwood, Kansas, 66205
Contact information: clinicaltrialdisclosure@jazzpharma.com
Kentucky Locations:
Norton Cancer Institute
Michael Driscoll, MD
Principal Investigator
Email: GI-NCIResearch@nortonhealthcare.org
Phone: +1 502-629-2500
Louisiana Locations:
Ochsner Clinic Foundation New Orleans
Location: New Orleans, Louisiana, 70121
Contact information: clinicaltrialdisclosure@jazzpharma.com
Massachusetts Locations:
Tufts Medical Center
Location: Boston, Massachusetts, US 2111
Contact information: clinicaltrialdisclosure@jazzpharma.com
Michigan Locations:
Henry Ford Health System
Location: Detroit, Michigan, US 48202
Contact information: clinicaltrialdisclosure@jazzpharma.com
University of Michigan
Vaibhav Sahai
Principal Investigator
UM Cancer Answerline Nurses
Study Coordinator
Email: CancerAnswerLine@med.umich.edu
Phone: +1 800-865-1125
Minnesota Locations:
Minnesota Oncology Hematology, P.A.
Location: Maple Grove, Minnesota, US 55369
Contact information: clinicaltrialdisclosure@jazzpharma.com
New Jersey Locations:
Atlantic Health System/Morristown Medical Center
Location: Morristown, New Jersey, US 7960
Contact information: clinicaltrialdisclosure@jazzpharma.com
New York Locations:
James J. Harding Memorial Sloan Kettering Cancer
James J. Harding, MD
Principal Investigator
Location: New York, New York, US 10022
Phone: +1 332-456-7016
Contact information: clinicaltrialdisclosure@jazzpharma.com
Laura and Isaac Perlmutter Cancer Center at NYU Langone – Ambulatory Care Center
Spencer
Principal Investigator
Location: New York, New York, US 10016
Contact information: clinicaltrialdisclosure@jazzpharma.com
Tennessee Locations:
Sarah Cannon Research Institute (SCRI) Oncology
Meredith Pelster, MD
Principal Investigator
Phone: +1 844-482-4812
Texas Locations:
Texas Oncology – DFW
Location: Dallas, Texas, US 75246
Contact information: clinicaltrialdisclosure@jazzpharma.com
The University of Texas MD Anderson Cancer Center
Location: Houston, Texas, US 77030
Contact information: clinicaltrialdisclosure@jazzpharma.com
University of Texas Southwestern Medical Center
Location: Dallas, Texas, US 75390
Contact information: clinicaltrialdisclosure@jazzpharma.com
South Carolina Locations:
Saint Francis Cancer Center Greenville
Yang
Principal Investigator
Location: Greenville, South Carolina, 29607
Contact information: clinicaltrialdisclosure@jazzpharma.com
Virginia Locations:
Virginia Commonwealth University, VCU Health
Location: Richmond, Virginia, US 23219
Contact information: clinicaltrialdisclosure@jazzpharma.com
